Post hoc analysis of REACH2 studied the impact of early intervention on CR1
REACH2: Timing by treatment initiation

aThis post hoc analysis examined ORR (CR + PR) at day 28, BOR at any time, and DOR between early (within 0-3 days of steroid-refractory aGVHD), late (4-6 days for ruxolitinib vs ruxolitinib and ≥4 days for ruxolitinib vs control therapy analyses), and very late (≥7 days) study treatment initiation. Subgroups were defined based on time from SR aGVHD to randomization. DOR was defined as the time from first response to aGVHD progression or the addition of new systemic therapy for aGVHD; competing risks were the onset of chronic GVHD or death without progression of aGVHD.1
bFor the ruxolitinib vs control therapy analysis, OR and 95% CI for ORR (or BOR) were calculated using the stratified Cochran-Mantel-Haenszel test. For the ruxolitinib vs ruxolitinib analysis, OR and 95% CI for ORR (or BOR) were calculated using Wald confidence limits.1
Post hoc analysis of REACH2 study: impact of early intervention on DOR with Jakafi1

aDOR was analyzed using the Kaplan-Meier method.1
Chart is from Blood, Socié G, Xue Z, Bhatt V, et al, Early versus late treatment with ruxolitinib in patients with steroid-refractory acute graft-versus-host disease: a post hoc analysis from the randomized phase 3 REACH2 study, 2022;140(suppl 1):4765-4766. Copyright © 2022 The American Society of Hematology. Reprinted with permission from The American Society of Hematology.
Competing risks include death without prior observation of aGVHD progression and onset of chronic GVHD.1
Review the primary endpoint from REACH2
Grade 2 patients on Jakafi had ~3x higher ORR2
REACH2: For all grades studied, ORRs at day 28 were higher with Jakafi vs control therapy2
REACH2: ORR at Day 28 (Primary Endpoint)2,a
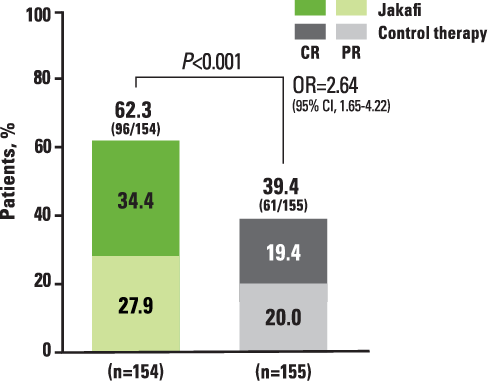
aDefined as the proportion of patients who had a CR or PR as compared with baseline organ staging without the use of additional systemic therapy for aGVHD.2
*CR is defined as a score of 0 for aGVHD grading in all evaluable organs, indicating complete resolution of all signs and symptoms of aGVHD in all evaluable organs without administration of additional systemic therapies for any earlier progression, mixed response, or nonresponse of aGVHD.2
REACH2 Subgroup Analysis: ORR at Day 28 by aGVHD Grades2

aGVHD=acute graft-versus-host disease; CI=confidence interval; CR=complete response; OR=odds ratio; ORR=overall response rate; PR=partial response; REACH=Ruxolitinib in patiEnts with refrACtory graft-versus-Host disease after allogeneic stem cell transplantation.
Study design
Jakafi was approved by the FDA for SR aGVHD based on positive results from the REACH1 study3
REACH1 Study Design4,5
REACH2 Study Design2
Phase 2 studya
Phase 3 studyc

REACH2 data are not included in the Jakafi Prescribing Information.
aPatients had grade 2 to 4 aGVHD, as defined according to MAGIC criteria, that occurred after allogeneic hematopoietic stem cell transplantation.4
b22 patients were not included in the efficacy analysis because they received 2 or more prior anti-GVHD therapies (n=12) or did not receive an adequate dose of corticosteroids (n=10). All 71 patients were included in the safety analysis.4,5
cThe REACH2 study was conducted in 105 centers across 22 countries.2
dCrossover from control therapy to Jakafi therapy was permitted if patients did not have a response at day 28 or if they had a loss of response thereafter and received additional systemic therapy, and they did not have signs of chronic GVHD.2
eControl therapy was chosen by the investigator at the time of randomization (antithymocyte globulin, extracorporeal photopheresis, mesenchymal stromal cells, low-dose methotrexate, mycophenolate mofetil, everolimus, sirolimus, etanercept, or infliximab).2
Definition of SR aGVHD in the REACH studies2,5
In both studies, SR aGVHD was defined as disease progression after 3 days of high-dose systemic steroid treatment, lack of improvement after 7 days of such treatment, or failure to successfully taper steroid
- REACH1 also specified patients as steroid refractory if they began low-dose steroids for skin or skin plus upper GI GVHD and developed GVHD in a new organ
- Failure to taper was defined in REACH1 as the inability to achieve a 50% taper of steroid dose, and in REACH2 as the need to increase to ≥2 mg/kg/day methylprednisolone or inability to taper below 0.5 mg/kg/day for at least 7 days
aGVHD=acute graft-versus-host disease; BID=twice daily; GI=gastrointestinal; MAGIC=Mount Sinai Acute GVHD International Consortium; ORR=overall response rate; REACH=Ruxolitinib in patiEnts with refrACtory graft-versus-Host disease after allogeneic stem cell transplantation; SR=steroid refractory.
aGVHD=acute graft-versus-host disease; BOR=best overall response; CI=confidence interval; CR=complete response; DOR=duration of response; GI=gastrointestinal; OR=odds ratio; ORR=overall response rate; PR=partial response; REACH=Ruxolitinib in patiEnts with refrACtory graft-versus-Host disease after allogeneic stem cell transplantation; SR=steroid refractory.
References: 1. Socié G, Xue Z, Bhatt V, Galvin J, Mohty M. Early versus late treatment with ruxolitinib in patients with steroid-refractory acute graft-versus-host disease: a post hoc analysis from the randomized phase 3 REACH2 study. Blood. 2022;140(suppl 1):4765-4766. 2. Zeiser R, von Bubnoff N, Butler J, et al; for the REACH2 Trial Group. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800-1810. Supplementary appendix available at: https://www.nejm.org/doi/10.1056/NEJMoa1917635. 3. Data on file. Incyte Corporation. Wilmington, DE. 4. Jakafi Prescribing Information. Wilmington, DE: Incyte Corporation. 5. Jagasia M, Perales MA, Schroeder MA, et al; for the REACH1 Study Group. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739-1749. Supplementary appendix available at: https://ashpublications.org/blood/article/135/20/1739/452638/Ruxolitinib-for-the-treatment-of-steroid.

