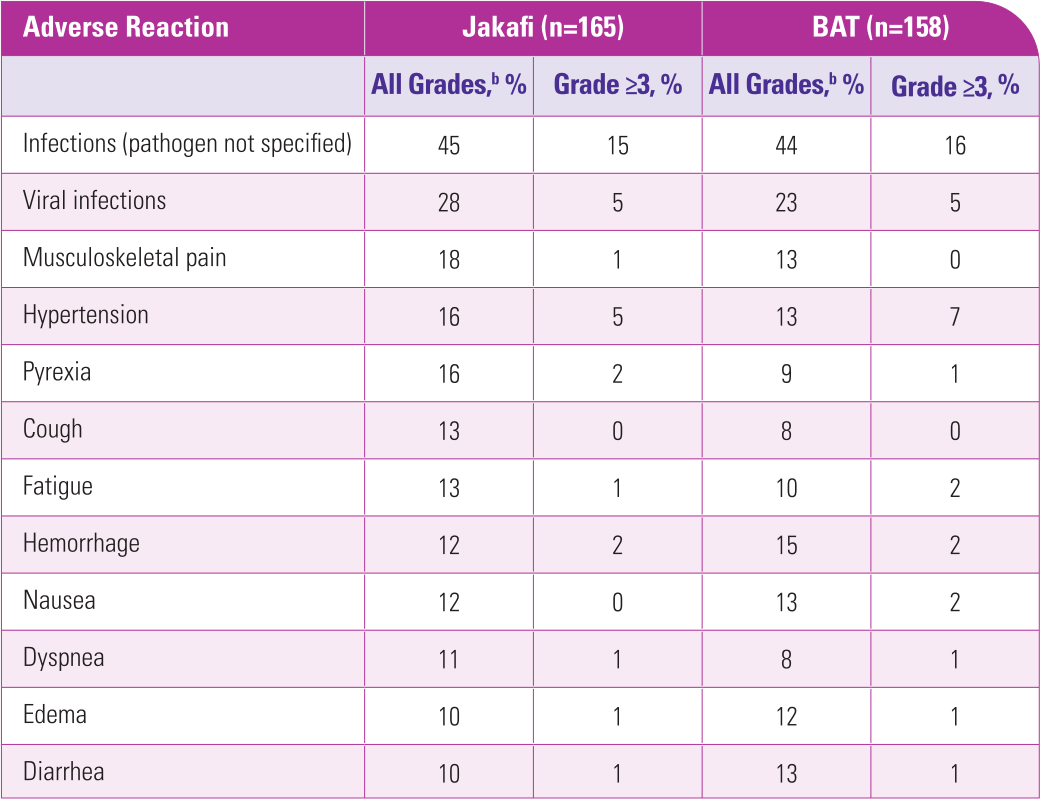

aSafety data from the Jakafi Prescribing Information.
bNational Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03.1


aSafety data from the Jakafi Prescribing Information.
bPresented values are worst-grade values regardless of baseline.1
cNational Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03.1


aSafety population: all patients who received ≥1 dose of study treatment.2
bInfections were classified by type at the investigator’s discretion by using an infection-specific grading system predictive of mortality that was developed for and validated in allogeneic stem cell transplant recipients based on the criteria provided in the protocol.2
ALT=alanine transaminase; AST=aspartate transaminase; BAT=best available therapy; REACH=Ruxolitinib in patiEnts with refrACtory graft-versus-Host disease after allogeneic stem cell transplantation.
References: 1. Jakafi Prescribing Information. Wilmington, DE: lncyte Corporation. 2. Zeiser R, Polverelli N, Ram R, et al; for the REACH3 Investigators. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385(3):228-238. Supplementary appendix available at: https://www.nejm.org/doi/full/10.1056/NEJMoa2033122. 3. Zeiser R, Russo D, Ram R, et al. Ruxolitinib in patients with chronic graft-versus-host disease: 3-year final analysis of efficacy and safety from the phase III REACH3 study. Presented at: 65th American Society of Hematology Annual Meeting and Exposition; December 9-12, 2023; San Diego, CA. Session 722.

