Dose optimization is key to maintaining the balance between safety and efficacy
For adults with intermediate- or high-risk MF, the recommended starting doses are based on platelet counts1
A CBC, including platelet count, must be performed before initiating Jakafi® (ruxolitinib)1
- Platelet count of 50 to <100 × 109/L: 5 mg BID
- Platelet count of 100 to 200 × 109/L: 15 mg BID
- Platelet count >200 × 109/L: 20 mg BID

Jakafi is also available in 10-mg and 25-mg tablets.
Special populations: Please refer to the Full Prescribing Information for starting dose and other dose modifications, and for when to avoid treatment in patients with renal or hepatic impairment and in those receiving concomitant strong CYP3A4 inhibitors or fluconazole.
Monitoring patients after initiation of Jakafi is essential, especially during the first 12 weeks of therapy1
- A CBC and platelet count must be performed every 2 to 4 weeks until doses are stabilized, and then as clinically indicated. Doses may be titrated based on safety and efficacy
- 70% of patients receiving Jakafi in COMFORT-I required a dose adjustment in the first 12 weeks of therapy2
Individualize dosing of Jakafi to optimize the balance between safety and efficacy1
Managing anemia and thrombocytopenia
- In COMFORT-I, grades 3 and 4 thrombocytopenia or anemia occurred in 13% and 45% of patients receiving Jakafi, respectively. All grades of thrombocytopenia or anemia occurred in 70% and 96% of patients receiving Jakafi, respectively1,3
- Dose modifications, temporarily withholding Jakafi, and/or transfusions may be required for patients developing anemia or thrombocytopenia1
- Interrupt treatment for bleeding, neutropenia (ANC <0.5 × 109/L), or thrombocytopenia (based on starting platelet count)1
ANC=absolute neutrophil count; CBC=complete blood count; COMFORT=COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment; Hb=hemoglobin; MF=myelofibrosis.
There are no contraindications for use of Jakafi, including in patients with anemia1
In COMFORT-I, 46% of patients receiving Jakafi had anemia at baseline; among these patients, mean Hb was 9.2 g/dL (range, 6.6 g/dL to 13.7 g/dL)4
Dose may be increased in the case of an insufficient response1
Efficacy based on titrated dose

This material is under a CC BY-NC License and is the property of the Ferrata Storti Foundation. © 2023 Ferrata Storti Foundation. All rights reserved.
- Doses may be increased if the response is insufficient and platelet and neutrophil counts are adequate and treatment has not been reduced or interrupted in the prior 4 weeks1
- Doses should not be increased during the first 4 weeks of therapy and not more frequently than every 2 weeks1
- Discontinue Jakafi if there is no spleen size reduction or symptom improvement after 6 months of therapy1
- In patients with starting platelet counts ≥100 × 109/L, based on limited clinical data, long-term maintenance at a 5-mg BID dose has not shown responses. Continued use at this dose should be limited to patients in whom the benefits outweigh the potential risks1
COMFORT=COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment.
Dosing in COMFORT-I1
- Treatment with Jakafi can cause thrombocytopenia, anemia, and neutropenia, which are each dose-related effects. Perform a pretreatment CBC and monitor CBCs every 2 to 4 weeks until doses are stabilized, and then as clinically indicated
- Manage thrombocytopenia by reducing the dose or temporarily interrupting Jakafi. Platelet transfusions may be necessary
- Patients developing anemia may require blood transfusions and/or dose modifications of Jakafi
- Severe neutropenia (ANC <0.5 × 109/L) was generally reversible by withholding Jakafi until recovery
Anemia clinical trial data
Dose modifications of Jakafi and/or blood transfusions may be required for patients developing anemia1
- In patients receiving Jakafi in the COMFORT studies, mean decreases in Hb levels reached a nadir of approximately 1.5 g/dL to 2.0 g/dL below baseline after 8 to 12 weeks of therapy and then gradually recovered to a new steady state that was approximately 1.0 g/dL below baseline1
- In COMFORT-I, 60% of patients treated with Jakafi and 38% of patients receiving placebo had RBC transfusions during randomized treatment1

From The New England Journal of Medicine, Verstovsek S, Mesa RA, Gotlib J, et al, A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis, 2012;366(9):799-807. Supplementary appendix available at: doi:10.1056/NEJMoa1110557. Copyright © 2012 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- In COMFORT-I, grade 3 or 4 anemia occurred in 45% of patients receiving Jakafi1
- <1% of patients receiving Jakafi in the COMFORT studies discontinued due to anemia or thrombocytopenia3

This material is under a CC BY-NC License and is the property of the Ferrata Storti Foundation. © 2023 Ferrata Storti Foundation. All rights reserved.
ANC=absolute neutrophil count; CBC=complete blood count; COMFORT=COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment; Hb=hemoglobin; RBC=red blood cell; SEM=standard error of the mean.
Thrombocytopenia clinical trial data
In patients with cytopenias, consider dose reductions, temporarily withholding Jakafi, or transfusions as clinically indicated.1
Monitor CBCs during treatment, beginning as early as weeks 2 to 4.1

aProtocol-mandated dose modifications occurred based on platelet count.
- In COMFORT-I, grade 3 or 4 thrombocytopenia occurred in 13% of patients receiving Jakafi1
- Initial reductions in Hb and platelets can occur in as early as 2 to 4 weeks3,4
- Dosing may need to be modified to avoid dose interruption, with the goal of achieving clinical benefit1
CBC=complete blood count; COMFORT=COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment; Hb=hemoglobin; LLN=lower limit of normal.
Appropriate dose management
In the case of a hematologic toxicity, including:
Thrombocytopenia
Discontinuations can be avoided by reducing the dose or temporarily withholding Jakafi.

In patients receiving Jakafi in the COMFORT studies, platelet counts and Hb levels generally stabilized after 8 to 12 weeks3,4
Anemia
Dose modifications of Jakafi and/or blood transfusions may be required for patients developing anemia.1
COMFORT=COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment; Hb=hemoglobin.
Interrupt Jakafi treatment for:
- Bleeding requiring intervention, regardless of current platelet count,
- Thrombocytopenia (platelet count <50 × 109/L), or
- Neutropenia (ANC <0.5 × 109/L)
Restarting after treatment interruption
- After recovery of platelet counts >50 × 109/L and ANC >0.75 × 109/L, dosing may be restarted
- The maximum allowable dose that may be used in restarting Jakafi after a previous interruption is shown below
Maximum restarting doses for Jakafi after safety interruption for thrombocytopenia

aMaximum doses are displayed. When restarting, begin with a dose ≥5 mg BID below the dose at interruption.
- Following treatment interruption for ANC <0.5 × 109/L, after ANC recovers to >0.75 × 109/L, restart dosing at the higher of 5 mg once daily or 5 mg BID below the largest dose in the week prior to the treatment interruption
ANC=absolute neutrophil count.
Increasing dose for insufficient response
In the case of an insufficient response, consider an increase in the dose if patient meets all of these criteria:
- Insufficient spleen reduction*
- Platelet count >125 × 109/L at 4 weeks and never <100 × 109/L
- ANC >0.75 × 109/L
*Failure to achieve a reduction from pretreatment baseline in either palpable spleen length of 50% or spleen volume of 35% as measured by CT or MRI.
Increase dose by increments of 5 mg BID to a maximum of 25 mg BID
Doses should not be increased during the first 4 weeks of therapy and should not be increased more frequently than every 2 weeks.
Discontinue Jakafi if there is no spleen size reduction or symptom improvement after 6 months of therapy.
ANC=absolute neutrophil count; CT=computed tomography; MRI=magnetic resonance imaging.
Appropriate dose management
In the case of a hematologic toxicity, including:
Thrombocytopenia
Discontinuations can be avoided by reducing the dose or temporarily withholding Jakafi.

In patients receiving Jakafi in the COMFORT studies, platelet counts and Hb levels generally stabilized after 8 to 12 weeks3,4
Anemia
Dose modifications of Jakafi and/or blood transfusions may be required for patients developing anemia.1
COMFORT=COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment; Hb=hemoglobin.
Interrupt Jakafi treatment for:
- Bleeding requiring intervention, regardless of current platelet count,
- Thrombocytopenia (platelet count <50 × 109/L), or
- Neutropenia (ANC <0.5 × 109/L)
Restarting after treatment interruption
- After recovery of platelet counts >50 × 109/L and ANC >0.75 × 109/L, dosing may be restarted
- The maximum allowable dose that may be used in restarting Jakafi after a previous interruption is shown below
Maximum restarting doses for Jakafi after safety interruption for thrombocytopenia

aMaximum doses are displayed. When restarting, begin with a dose ≥5 mg BID below the dose at interruption.
- Following treatment interruption for ANC <0.5 × 109/L, after ANC recovers to >0.75 × 109/L, restart dosing at the higher of 5 mg once daily or 5 mg BID below the largest dose in the week prior to the treatment interruption
ANC=absolute neutrophil count.
Increasing dose for insufficient response
In the case of an insufficient response, consider an increase in the dose if patient meets all of these criteria:
- Insufficient spleen reduction*
- Platelet count >125 × 109/L at 4 weeks and never <100 × 109/L
- ANC >0.75 × 109/L
*Failure to achieve a reduction from pretreatment baseline in either palpable spleen length of 50% or spleen volume of 35% as measured by CT or MRI.
Increase dose by increments of 5 mg BID to a maximum of 25 mg BID
Doses should not be increased during the first 4 weeks of therapy and should not be increased more frequently than every 2 weeks.
Discontinue Jakafi if there is no spleen size reduction or symptom improvement after 6 months of therapy.
ANC=absolute neutrophil count; CT=computed tomography; MRI=magnetic resonance imaging.
Decreasing dose for hematologic toxicity
Reduce the dose of Jakafi in patients with platelet counts <35 × 109/L.

Interrupt Jakafi treatment for:
- Bleeding requiring intervention, regardless of current platelet count,
- Platelet counts <25 × 109/L, or
- ANC <0.5 × 109/L
Restarting after treatment interruption
Restart dosing after recovery of platelet counts to >35 × 109/L and ANC <0.75 × 109/L at the higher of:
- 5 mg once daily or
- 5 mg BID below the largest dose in the week prior to the decrease in platelet count below 25 × 109/L or ANC below 0.5 × 109/L that led to dose interruption
ANC=absolute neutrophil count.
The recommended starting dose in MF for patients with a starting platelet count of 50 to <100 x 109/L is 5 mg BID
Increasing dose for insufficient response
In the case of an insufficient response, consider an increase in the dose only after the first 4 weeks of therapy and not more frequently than every 2 weeks if patients meet all of these criteria:
- Insufficient spleen reduction*
- Platelet count has remained ≥40 × 109/L at 4 weeks and has not decreased by >20% in the prior 4 weeks
- ANC >1.0 × 109/L
- No dose reduction or interruption for an AE or hematological toxicity in the prior 4 weeks
*Failure to achieve a reduction from pretreatment baseline in either palpable spleen length of 50% or spleen volume of 35% as measured by CT or MRI.
Increase dose by increments of 5 mg daily to a maximum of 10 mg BID
Continuation of treatment for more than 6 months should be limited to patients in whom the benefits outweigh the risks.
Discontinue Jakafi if there is no spleen size reduction or symptom improvement after 6 months of therapy.
AE=adverse event; ANC=absolute neutrophil count; CT=computed tomography; MF=myelofibrosis; MRI=magnetic resonance imaging.
Decreasing dose for hematologic toxicity
Reduce the dose of Jakafi in patients with platelet counts <35 × 109/L.

Interrupt Jakafi treatment for:
- Bleeding requiring intervention, regardless of current platelet count,
- Platelet counts <25 × 109/L, or
- ANC <0.5 × 109/L
Restarting after treatment interruption
Restart dosing after recovery of platelet counts to >35 × 109/L and ANC <0.75 × 109/L at the higher of:
- 5 mg once daily or
- 5 mg BID below the largest dose in the week prior to the decrease in platelet count below 25 × 109/L or ANC below 0.5 × 109/L that led to dose interruption
ANC=absolute neutrophil count.
The recommended starting dose in MF for patients with a starting platelet count of 50 to <100 x 109/L is 5 mg BID
Increasing dose for insufficient response
In the case of an insufficient response, consider an increase in the dose only after the first 4 weeks of therapy and not more frequently than every 2 weeks if patients meet all of these criteria:
- Insufficient spleen reduction*
- Platelet count has remained ≥40 × 109/L at 4 weeks and has not decreased by >20% in the prior 4 weeks
- ANC >1.0 × 109/L
- No dose reduction or interruption for an AE or hematological toxicity in the prior 4 weeks
*Failure to achieve a reduction from pretreatment baseline in either palpable spleen length of 50% or spleen volume of 35% as measured by CT or MRI.
Increase dose by increments of 5 mg daily to a maximum of 10 mg BID
Continuation of treatment for more than 6 months should be limited to patients in whom the benefits outweigh the risks.
Discontinue Jakafi if there is no spleen size reduction or symptom improvement after 6 months of therapy.
AE=adverse event; ANC=absolute neutrophil count; CT=computed tomography; MF=myelofibrosis; MRI=magnetic resonance imaging.
Dose modification for bleeding1
Interrupt treatment for bleeding requiring intervention regardless of current platelet count. Once the bleeding event has resolved, consider resuming treatment at the prior dose if the underlying cause of bleeding has been controlled. If the bleeding event has resolved but the underlying cause persists, consider resuming treatment with Jakafi at a lower dose.
Dose modifications for special populations1
Dose modifications in patients with renal or hepatic impairment, or when coadministered with strong CYP3A4 inhibitors or fluconazole

Patients with ESRD on dialysis
- The recommended starting dose in patients with MF who have ESRD (CLcr <15 mL/min) and are on dialysis is:
- 15 mg once after a dialysis session for patients with a platelet count between 100 × 109/L and 200 × 109/L
- 20 mg once after a dialysis session for patients with a platelet count >200 × 109/L
- Additional dose modifications should be made with frequent monitoring of safety and efficacy
- Avoid use of Jakafi in patients with ESRD (CLcr <15 mL/min) not requiring dialysis
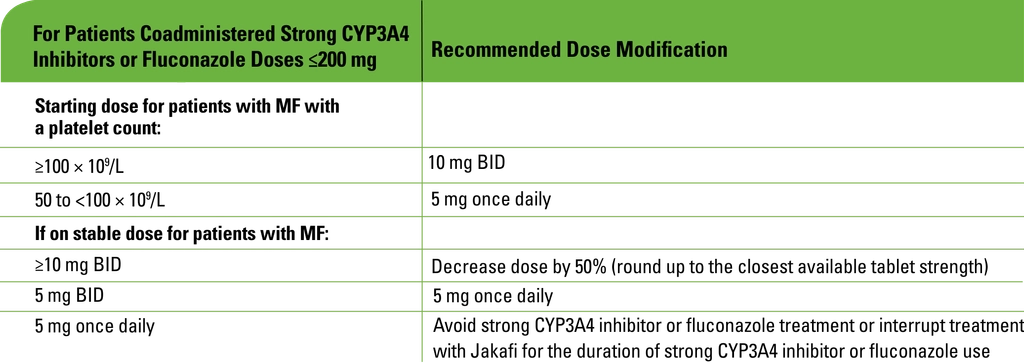
Concomitant use with strong CYP3A4 inhibitors or fluconazole
- Modify the dose of Jakafi when coadministered with strong CYP3A4 inhibitors and fluconazole doses of ≤200 mg
- Avoid the use of fluconazole doses >200 mg daily with Jakafi
- Additional dose modifications should be made with frequent monitoring of safety and efficacy
BID=twice daily; CLcr=creatinine clearance; ESRD=end-stage renal disease; MPN=myeloproliferative neoplasm; OS=overall survival; SVR=spleen volume reduction.
References: 1. Jakafi [package insert]. Wilmington, DE: Incyte Corporation. 2. Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98(12):1865-1871. Supplementary appendix available at: https://haematologica.org/article/view/6861. 3. Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. Supplementary appendix available at: https://www.nejm.org/doi/full/10.1056/nejmoa1110557. 4. Data on file. Incyte Corporation. Wilmington, DE.

