Jakafi® (ruxolitinib) safety and tolerability data: JUMP expanded-access study1,2
JUMP study design1
- JUMP (JAK Inhibitor RUxolitinib in Myelofibrosis Patients) was a single-arm, open-label, phase 3b, expanded-access study that enrolled adult patients with primary or secondary MF classified as intermediate-1-, intermediate-2-, or high-risk MF. Patients with intermediate-1-risk MF were required to have a palpable spleen (≥5 cm from the costal margin)
- The study enrolled 2233 patients. At study entry, 835 patients had intermediate-1-risk MF, 755 patients had intermediate-2-risk MF, and 194 patients had high-risk MF*†
- The primary endpoint was assessment of Jakafi safety and tolerability by the frequency, duration, and severity of AEs. Additional endpoints included the proportion of patients with ≥50% reduction in palpable spleen length

aDIPSS scores were determined using patient characteristics at baseline. Results are based on a 07/05/17 cutoff date.1

aDIPSS scores were determined using patient characteristics at baseline. Results are based on a 07/05/17 cutoff date.1
In the overall JUMP population, the safety profile was generally consistent with previous reports for Jakafi
- 58% of all enrolled patients (n=1283) completed treatment per protocol1
- Completing treatment was defined as undergoing treatment for up to 24 months after the last patient's first visit or transitioning to a commercial drug
- The most common AEs leading to discontinuation were thrombocytopenia (3%, 58/1784) and anemia (2%, 35/1784)2
Reduction in spleen length by disease severity
JUMP efficacy data
- At week 96, 67% (423/636) of efficacy-evaluable patients achieved a ≥50% reduction from baseline in palpable spleen length1
Intermediate-1–risk MF and intermediate-2–risk MF to high-risk MF1,2
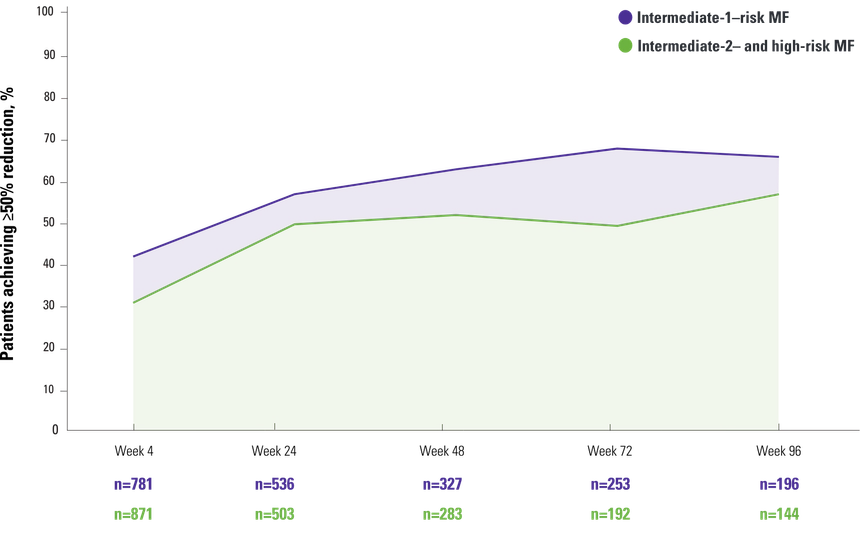
Intermediate-1–risk MF2


One question I am often asked is … would I initiate Jakafi for my intermediate-1–risk patients or those appropriate patients who are earlier in the course of the disease? And my answer is yes.
*DIPSS scores were determined using patient characteristics at baseline. Results are based on a 07/05/17 cutoff date.1
†Patients who were not classified into a risk group (n=389) and patients with low-risk MF (n=60) are not included in the data shown.1
AE=adverse event; DIPSS=Dynamic International Prognostic Scoring System; MF=myelofibrosis; OS=overall survival.
References: 1. Al-Ali HK, Griesshammer M, Foltz L, et al. Primary analysis of JUMP, a phase 3b, expanded-access study evaluating the safety and efficacy of ruxolitinib in patients with myelofibrosis, including those with low platelet counts. Br J Haematol. 2020;189(5):888-903. 2. Data on file. Incyte Corporation. Wilmington, DE.

